-
Intergence Powers Tendring District Council to Achieve Highest Cyber Security Rating
We are delighted to announce that Tendring District Council have just received an ‘EPIC’ cyber security rating for their email domain from the Cyber Technical Advisory Group (CTAG) in a recent audit. This prestigious rating underscores the council's commitment to maintaining the highest standards of cyber security and their partnership with Intergence played a big part in this achievement working collaboratively alongside Tendring’s in-house team.
added by Intergence 1 min Read -
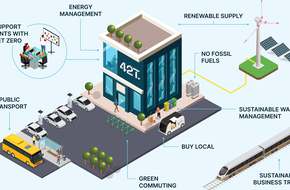
42T signs SBTi commitment for emissions reduction
42 Technology (42T) has today underscored its company-wide commitment to sustainability and net-zero by signing a commitment to emissions reduction near-term targets with the Science Based Targets initiative (SBTi), the widely accepted benchmark for decarbonisation targets aligned to the Paris Agreement.
added by 42T 2 min Read -
Cambridge tech firm pitches to South Korea’s biggest maritime corporations
Seven UK companies offering innovative technologies for the maritime sector, including Cambridge-headquartered AI pioneer Darktrace, have pitched their offerings to five of South Korea’s largest shipbuilding and ship owning corporations during a trade mission to the UK this week.
added by Intralink 3 min Read -
Cambridge Wireless Announces Michaela Eschbach as New CEO
Cambridge Wireless is delighted to announce the appointment of Michaela Eschbach as our new Chief Executive. Michaela will commence her new role on 13th May 2024.
added by Cambridge Wireless 1 min Read -
Comparing the Cost between Injection Moulding and 3D Printing
Learn about the cost drivers for both services as parts and products move from prototyping to production.
added by Protolabs 4 min Read -
The EBCam Employee Benefits and Rewards Survey is live for 2024
The EBCam Employee Benefits and Rewards survey enables organisations to benchmark their offering against other comapniess, enabling accurate and up to date market information to support any decisions to add, remove or amend any benefits or rewards for your team.
added by EBCam Ltd 1 min Read -
ForefrontRF make the finals in the 2024 Cambridge Independent Science and Technology Awards
Forefront RF, a fabless semiconductor company based in Cambridge, UK, has been named a finalist in the Cambridge Independent Science and technology awards 2024 under the “One to Watch” category. The awards are in their seventh year and 2024 and this year’s winners will be revealed at a ceremony taking place atHinxton Hall Conference Centre on the 16th May.
added by Forefront RF Ltd 1 min Read -
Sookio launches new podcast series, Communication Untangled
Join host Sue Keogh and guests from the worlds of design, communications, and behavioural psychology for the podcast that explores the many facets of communication that influence our behaviour - but often go unnoticed. From menu design to motorway typography, from brand guidelines to the colours that make us click, we’ll shine a light on techniques you can apply to get across critical messages in your marketing, business and brand.
added by Sookio 1 min Read -
Spring forward with a 10% discount, Lessons in negotiation, Sardinian secrets to longevity & Italy’s booming economy - Newsletter 2024
Spring has finally arrived, and with it, a bustling season of European Work Council (EWC) meetings. As an interpreter who has worked with over 20 companies on their EWC negotiations, I've learned a few valuable lessons that I share in this month's newsletter. In this month's newsletter, you'll also find insights into Italy's booming economy, my tutoring service for busy executives, the secrets of Sardinian centenarians, and the importance of setting your own pace in business.
added by One Stop Language Solutions 1 min Read -
Introducing the ProShotG Endpoint Antenna
antennaPRO is pleased to announce the latest antenna innovation from Amphenol Procom, the ProShotG. The ProShotG is an endpoint antenna designed for both fixed and mobile applications.
added by antennaPRO 1 min Read -
Intellectual Property Firm Appleyard Lees Appoints New Partner
Appleyard Lees is delighted to announce that patent attorney, Paul Beynon (MEng, CPA, EPA), joined the partnership on 1 April 2024.
added by Appleyard Lees IP LLP 1 min Read -
Protolabs and Hutchinson collaborate on the first Italian electric supercar
Designing components for a super car means thinking big, delivering exceptional performance, and executing a flawless design. Learn how Hutchinson worked with Protolabs to injection mould a battery pack cooling system in just 5 weeks for an Italian electric supercar.
added by Protolabs 3 min Read -
“Master Your Business Finances” (17th April 2024-Online Event)
Are you worried about not "Uncovering Your Unknowns"? Then this free workshop is for you. Hosting at 1 PM on 17th April 2024 (GMT)
added by Watermill Accounting/Innovation Credit 1 min Read -
Chinese consumer tech companies’ visit to Cambridge - innovation and exchange of ideas
VR, AI, IoT, industrial innovation, just some of the topics we covered in one day – what a productive visit for these Chinese consumer tech companies in Cambridge!
added by Crayfish.io 1 min Read -
Black Talent and Leadership in STEM shortlisted for Cambridge Independent Award
We are delighted to announce that Black Talent and Leadership in STEM has been shortlisted for the STEM Initiative of the Year award by the Cambridge Independent Science and Technology Awards 2024. Winners will be announced at a special awards ceremony taking place on 16th May 2024 at Hinxton Hall at the Wellcome Genome Campus,
added by Black Talent & Leadership in STEM 1 min Read -
The UK's Product Security and Telecommunications Infrastructure (PTSI) Act: What our customers using Cellular/ Wifi/ Bluetooth modules need to know.
As a specialist provider of positioning and wireless communication modules for IoT applications, Alpha Micro is committed to keeping its customers informed about relevant regulations and their potential impact. The recently enacted Product Security and Telecommunications Infrastructure (PTSI) Act in the UK introduces new cybersecurity requirements for manufacturers, importers, and distributors of internet-connected products. This blog aims to provide a clear understanding of the Act and its potential implications for their businesses.
added by Alpha Micro Components 3 min Read -
Cambridge spinoff selected for Government support in Japan
An advanced materials spinoff from Cambridge University is amongst four innovative UK firms to have been selected to receive support from the UK-APAC Tech Growth Programme to accelerate its plans for expansion in Japan. All four companies offer a range of technologies identified as of high interest to Japanese corporations.
added by Intralink 2 min Read -
Covnetics - FPGA Resource
Covnetics - FPGA resource available (for a limited time)
added by Covnetics 1 min Read -
EMC&CI 2024 Conference programme officially launched
Join us at the EMC&CI 2024 Conference on May 22nd and 23rd for two days of expert insights on Electromagnetic Compatibility (EMC). Explore practical compliance and advanced product design with industry leaders. Secure your spot now!
added by Mach One Design Ltd 1 min Read -
Material Hardness Testing of CNC Machined Parts
Ensuring the right level of hardness for your CNC machined part.
added by Protolabs 4 min Read -
How to get the best out of your Interpreter at a Live Event
One of my favourite jobs this year was interpreting a live session at the FT’s Business of Football Summit. Aurelio De Laurentiis, a veteran movie producer who has transformed the fortunes of the football club SSC Napoli since he saved it from bankruptcy in 2004, was interviewed on stage in Italian by an FT journalist. The experience inspired me to share some quick tips for Cambridge Wireless readers who might be organising a conference or live event with multi-lingual guests or speakers.
added by One Stop Language Solutions 2 min Read -
Medical innovations, Sustainability, Agritech and AI dominate #21toWatch 2024 Awards
The 6th annual #21toWatch innovation awards have been unveiled at an awards ceremony at The Bradfield Centre in Cambridge, the UK Centre for Science, Technology and Innovation.
added by cofinitive Ltd 7 min Read -
Revolutionizing Outdoor Enclosure Technology
Revolutionizing Outdoor Enclosure Technology: GTT Wireless IP67 mSmart-box® is 'Project Critical' Over 101,000 visitors to the Mobile World Congress 2024 gathered in Barcelona last week to shape, debate and celebrate the future of connectivity. NewEdge Signal Solutions featured the GTT Wireless ground breaking 'm-Smart Box' enclosure ensuring unparalleled protection for their ADI powered radio unit, NewEdge SR-30N78 - Outdoor O-RAN 7.2x50 small cell outdoor 4T4R, n78.
added by GTT Wireless Ltd 2 min Read -
5 Things to consider when hiring an Interpreter
Is your business looking to use interpretation services for the first time? …there is a lot to consider! To help you make the right decision, I have compiled the Top 5 things to consider when hiring an interpreter, a guide to ensure you get the best person for your project.
added by One Stop Language Solutions 2 min Read -
Mpirical Launches Lab Environment for Hands-on Training
This week at MWC Barcelona 2024, global telecoms training company, Mpirical is thrilled to introduce a further hands-on learning tool to complement its extensive video learning resources for the telecoms industry.
added by Mpirical 1 min Read -
Protolabs helps Arrow Lake develop ozone system that cuts costs by 20%
Clean by ozone. It’s a term many may not be familiar with but for Arrow Lake they are on a mission to develop the world’s most efficient ozone technology and energy-efficient solutions with help from Protolabs.
added by Protolabs 5 min Read -
European Commission’s plan to update the European Works Councils Directive
Have you heard about the European Commission’s plan to update the European Works Councils Directive? It’s a big step forward for social dialogue in the EU!
added by One Stop Language Solutions 1 min Read -
“Master Your Business Finances” (13th March 2024-Online Event)
Are you worried about not "Uncovering Your Unknowns"? Then this free workshop is for you. Hosting at 2 PM on 13th March 2024 (GMT)
added by Watermill Accounting/Innovation Credit 1 min Read -
Black Talent and Leadership in STEM Programme welcomes Marks & Clerk as Consortium Member
We are pleased to announce that leading international intellectual property firm Marks & Clerk has joined the Black Talent and Leadership Programme as a Consortium member of the ‘Be the Change’ initiative which addresses the underrepresentation of Black Talent in STEM.
added by Black Talent & Leadership in STEM 2 min Read -
Alpha Micro and The Antenna Company Join Forces to Propel Cellular and Short-Range Connectivity Solutions
Alpha Micro will become The Antenna Company’s Exclusive Distributor for UK and Ireland.
added by Alpha Micro Components 2 min Read -
International Mother Language Day
🌍 Today marks International Mother Language Day, a significant occasion championed by UNESCO to celebrate linguistic diversity, cultural richness, and the promotion of multilingualism across the globe.
added by One Stop Language Solutions 1 min Read -
Water-sensing technology company Infersens launches first commercially available solution at World Water-Tech Innovation Summit
Cambridge-based Infersens has launched Cortense®, its first commercially available solution to transform Legionella risk management and dramatically cut water waste.
added by cofinitive Ltd 2 min Read -
Infersens launches Cortense®
Water-sensing technology company Infersens launches first commercially available solution
added by Infersens Ltd 2 min Read -
Global tech leaders, innovators and investors unite as Cambridge Tech Week returns for 2024
Taking place from 9-13 September 2024, Cambridge Tech Week will showcase renowned research and innovation with the theme of ‘Innovate, Invest and Grow’
added by Cambridge Wireless 3 min Read -
Artificially Intelligent Cities?
How can AI help local authorities to create better, more sustainable places for their residents?
2 min Read -
When in Doubt, Go With the Flow - Proper Resin Flow
Designing excellent injection-moulded parts is dependent on many factors. Proper flow of the resin into the mould is one of them.
added by Protolabs 2 min Read -
Cambridge firms kick off commercial negotiations at SEMICON Korea
Government programme opens doors to Korean interest in innovative chip technologies
added by Intralink 2 min Read -
“Master Your Business Finances” (07th February 2024-Online Event)
Are you worried about not "Uncovering Your Unknowns"? Then this free workshop is for you. Hosting at 2 PM on 07th February 2024 (GMT)
added by Watermill Accounting/Innovation Credit 1 min Read -
Success for ip21’s client Borg and Overström’s SensorBeam® Technology wins ‘Best Dispense Innovation Award’
Borg and Overström's revolutionary SensorBeam® technology, a touchless water dispensing system using projection mapping, received the 'Best Dispense Innovation' Award at the Global Water Drinks Congress in Gleneagles, Scotland.
added by IP21 1 min Read -
New event for founders: Why you need an Exit Strategy now
Do you have a plan to exit your business? It’s never too early to start! Business exit strategy experts Yellowyoyo have teamed up with Innovate UK EDGE to deliver an essential event for founders that will set you on the road to maximising value, whether you plan to exit in five years or fifty years.
added by St John's Innovation Centre 1 min Read -
Anglia Bulgin Supplier of the Year
Anglia announces its Supplier of the Year Bulgin’s strong customer support wins them the award
added by Anglia 2 min Read -
Degree Apprenticeships at ARU Proudly Supporting National Apprenticeship Week 2024
Degree Apprenticeships at ARU Proudly Supporting National Apprenticeship Week 2024
added by Anglia Ruskin University 1 min Read -
Why the time is now for UK tech scaleups to enter Korea
Narai Kim from Intralink's Seoul office deep-dives into why South Korea is currently a key market to target for UK tech companies, following the Korean President's trip to London.
added by Intralink 5 min Read -
Protolabs tackles manufacturing skills gap with launch of inspirON academy
Digital manufacturer, Protolabs, has launched its inspirON academy. A series of free CPD-accredited online training courses, inspirON academy aims to tackle a widening skills gap by helping engineers at any stage in their career to increase their understanding of designing for injection moulding, 3D printing and CNC machining.
added by Protolabs 1 min Read -
Black Talent and Leadership in STEM Programme welcomes Arm as first consortium member
We are pleased to announce that semiconductor IP leader Arm has joined the Black Talent and Leadership Programme as the first Consortium member of the ‘Be the Change’ initiative which addresses the underrepresentation of Black talent in STEM.
added by Black Talent & Leadership in STEM 1 min Read -
Iprova launches 3GPP standards monitoring and contribution creation support.
Pioneering AI invention company Iprova has expanded its recently-launched Invention Studioevo platform offering to now include specialised functionality for members of the 3GPP standards project.
added by Iprova Ltd 2 min Read -
“Master Your Business Finances” (17th January 2024-Online Event)
Are you worried about not "Uncovering Your Unknowns"? Then this free workshop is for you. Hosting at 2 PM on 17th January 2024 (GMT)
added by Watermill Accounting/Innovation Credit 1 min Read -
Forefront RF recognised as “Rising Star” by Sifted
Backed by the Financial Times, Sifted is a leading media brand for the European start up community
added by Forefront RF Ltd 1 min Read -
Manufacturing Considerations for Plastic Prototyping
Discover if CNC machining, 3D printing or injection moulding is best for your plastic prototype.
added by Protolabs 6 min Read -
AI expert joins Iprova Advisory Board
Ex-Amazon artificial intelligence expert Dr Catherine Breslin joins Iprova's Advisory Board
added by Iprova Ltd 1 min Read -
5 Principles for Encouraging Your Supply Chain to Limit Carbon Emissions
Find out how to make your business more sustainable in 2024
added by Cambridge Wireless 4 min Read -
Developments in Motion Control Technology streamline Industrial Automation Design
Accurate motion control is the heart of modern industrial automation. Whether it's a robotic arm assembling intricate electronic components or a conveyor system managing the flow of goods, this technology plays a pivotal role in achieving seamless operations. It is like the invisible but ever attentive servant that delivers precisely, accurately and predictably the movements that the system mandates, bringing robots, CNC machines, and numerous other automated processes to life. The quality of its design has a huge impact on the speed, efficiency, quality and material consumption of the industrial process as a whole.
added by Anglia 6 min Read -
Beware of complacency in 2024
As we look to the year ahead, the supply chain looks healthier than it has for some time, with good levels of inventory overall, and shorter lead times than we’ve seen for some time. Customers should beware of complacency though, as the economic headwinds that are already visible will mean that this situation won’t last. Inflation continues to have a sustained impact on the economy. Although there is likely to be limited growth in the industrial sector, we expect other markets including those driven primarily by consumer spending to decline.
added by Anglia 5 min Read -
Intellectual Property Firm Appleyard Lees Appoints New Managing Partner
Bobby Smithson, Partner at leading intellectual property law firm Appleyard Lees, has been appointed as the firm’s Managing Partner, effective 1 January 2024.
added by Appleyard Lees IP LLP 1 min Read -
Preparing for the power of 6G
Enhanced connectivity is driving global innovation and improving efficiency, communication, and sustainability in almost every industry. As it expands its reach around the world, 5G is accelerating these developments and constantly generating new and exciting use cases. But with the path to 6G already in motion, what is the future of connectivity?
added by CGI 3 min Read -
CGI joins expert panel to launch Northern Ireland’s largest 5G Mobile Private Network testbed
On Wednesday 8 November 2023, CGI took part in the launch of Northern Ireland’s largest 5G testbed, which is set to supercharge smart manufacturing and education in the region
added by CGI 1 min Read -
CGI, Eutelsat OneWeb and Icomera to pilot improved broadband communications for train travel through seamless hybrid satellite and terrestrial networks
CGI (NYSE: GIB) (TSX: GIB.A) is running live trials to demonstrate how integrated low-earth orbit satellite and terrestrial communications networks can provide seamless, low latency broadband connectivity on Britain’s rail network.
added by CGI 2 min Read -
Data and AI: the key to improving network reliability and managing customer satisfaction
We all rely on broadband connectivity in our everyday lives, yet we so easily take it for granted. Whether it’s using social media to keep up with family and friends, or online shopping, banking, booking a table at our favourite restaurant, or even travelling by plane, Uber or train - we often don’t realise that this connectivity underpins so much of what we do. For businesses, it’s even more critical.
added by CGI 3 min Read -
CGI to deliver the largest 5G Private Network testbed in Northern Ireland in partnership with Digital Catapult and Nokia
In partnership with Nokia, we are delivering ground-breaking 5G testbed facilities in Northern Ireland set to supercharge smart manufacturing and education in the region over the next five years.
added by CGI 2 min Read -
Blurring the lines between Mobility and Fixed Wireless Access – ProEdge Antenna
We are thrilled to announce the launch of our revolutionary fixed wireless ProEdge antenna, designed for connectivity for fixed wireless access and mobility solutions alike!
added by antennaPRO 1 min Read
News
News and updates from our global community
Latest news from across the network
Subscribe to the CW newsletter
This site uses cookies.
We use cookies to help us to improve our site and they enable us to deliver the best possible service and customer experience. By clicking accept or continuing to use this site you are agreeing to our cookies policy. Learn more
Start typing and press enter or the magnifying glass to search

CW TEC 2024: Engineering AI for Critical Systems
CW TEC 2024 focuses on integrating AI in critical industries, highlighting this technology's potential and engineering challenges. Join us on 27 June at the Computer Laboratory, University of Cambridge
Book your place today
Cambridge Tech Week is back!
CTW returns for 2024 from 9 - 13 September in the heart of Cambridge.
Register your interest today
Cambridge Wireless connects the wireless community
Cambridge Wireless is a vibrant membership organisation for companies involved in the research, development and application of wireless and associated technologies.
Find out more about membership
CW Techsters is back for its third year
CW Techsters is a free programme that enables young people aged 16-22 to learn about the basics of technologies while challenging them to think about real-world issues
Find out more about CW Techsters
CW announces their participation in UK Telecoms Innovation Network
CW joins Digital Catapult,University of Bristol and West Midlands 5G to set up and oversee a new network dedicated to boosting creativity in the country’s telecoms supply chain
Find out more
CW Academy
Boost your business and marketing skills with our new training courses led by world-class product and technology specialists
find out more